
Manganese is an essential plant micronutrient which is a key component of many important metabolic processes. In particularly, it is crucial as an enzyme co-factor for the catalysis of many redox reactions in photosystem II and nitrogen assimilation. It is also essential for many reactions that allow plants to tolerate environmental stresses. Some typical symptoms of manganese deficiency include interveinal chlorosis or mottling and/or the browning of leaf margins, especially on young leaves. An extreme case is pictured above on rocket plants (Eruca sativa) growing in the Plater Bio glasshouse.
Unfortunately the symptoms of manganese deficiency are often indistinguishable from iron deficiency, and soil/tissue analysis is thus required to confirm the limiting nutrient. This problem is also further compounded by the fact that, in a similar situation to deficiencies in iron, manganese becomes unavailable to plants as the soil pH rises. Therefore, deficiencies in these two micronutrients are common in neutral to alkali soils. In fact, they are two of the most common nutrient deficiencies found in crops globally. For this reason, manganese deficiency is very common in ericaceous crops growing in alkaline soils. This includes many ornamentals but also cereals, especially barley, which needs a comparatively high soil pH.
Manganese deficiencies are also associated with the use of the herbicide glyphosate. Glyphosate (pictured below) sold under its tradename ‘Roundup’ is one of the most commonly used herbicides globally. Glyphosate directly inhibits the absorption of manganese from the soil and its subsequent translocation (10-50% reduction compared to normal capacity). The glyphosate anion also binds the manganese cation in the soil thus immobilizing it (AKA ‘lock up’). Therefore, manganese deficiencies can be a problem either when glyphosate is used as a pre-plant herbicide, spot-applied post-emergence herbicide, crop dessicant, or where genetically-modified glyphosate-resistant crops are cultivated. In these situations, manganese fertilizer rates need to be elevated by around 50% and chelated foliar applications are additionally required when the soil is alkaline or excessive glyphosate levels are present in the soil. It is not just the crop that is affected by glyphosate application, with beneficial soil microorganisms also harmed by glyphosate inhibiting their uptake and use of the essential manganese nutrient.
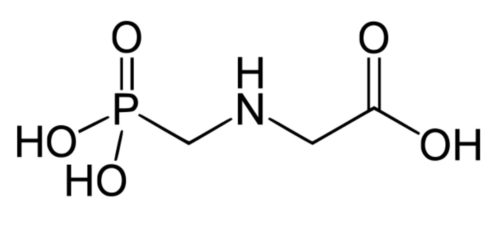
As is known for iron deficiencies, simply replacing the deficient mineral with a soil drench of a sulphate salt fertilizer is not an adequate solution to the problem as the iron/manganese will almost instantly be oxidised into unavailable forms once the acidic manganese sulphate solution reaches the alkaline soil. Even if the ionic manganese makes it into the plant, as manganese is largely xylem mobile, during times of stress manganese will become limited if transpiration slows as manganese cannot be remobilised for use in growing tissues. This is the reason the signs of manganese deficiency are seen first in young leaves. In order to overcome these problems foliar applications of chelated manganese are required to get the nutrient to where it is most needed, even in periods of stress by being phloem-mobile and by-passing soil-related issues. While this has traditionally been achieved by the use of synthetic aminopolycarboxylates chelates (e.g. EDTA), Plater Bio are taking an alternative approach with the use of neutralized organic acids in the form of Manganese Ammonium Citrate (pictured below). Our technical team have developed this unique product that has some major advantages over the manganese based products currently on the market.

Aminopolycarboxylates have questionable biodegradability and thus the synthetic molecule can build up in soil if used over successive growing cycles. Some of these chelates can also be too powerful at locking in the metal ion, so actual plant availability can be limited. In contrast, Manganese Ammonium Citrate is more in balance with natural processes as citrates are naturally used by plants to solubilize and transport micronutrients. Ammonium citrate chelates are also readily biodegraded in the Krebs cycle thus leaving no harmful residues.
Manganese Ammonium Citrate (MnAC) is naturally alkaline meaning it is less likely to oxidise in alkaline soils compared to EDTA-Mn and manganese sulphate, both of which are acidic.
An often-overlooked disadvantage of many manganese sulphate products on the market is that most have very low purity levels. This leads to sediment build up and discolouration. So, while Plater Bio purify our standard manganese sulphate to the level shown below on the left, you will also see products on sale to end users from other suppliers that look opaque and lack manganese sulphate’s classic pink colour (pictured right).

Because EDTA-Mn is an expensive product its use is largely restricted to high-value horticultural applications. However, it is often surprising how costly simple manganese sulphate products can be to arable end-users (e.g. barley farmers). At Plater Bio we are confident that our new Manganese Ammonium Citrate liquids and fully soluble spray dried powders will finally make it cost effective for broad acre arable farmers to access chelated manganese at similar costs per hectare.


-500-width.jpg)